SMA® ALTHÉRA®
ADVANCING THE DIETARY MANAGEMENT OF COW’S MILK ALLERGY
- Extensively hydrolysed, hypoallergenic, whey-based formula (eHF) for the effective first line dietary management of the majority (~90%) of infants with cows’ milk allergy (CMA) and other conditions where an extensively hydrolysed formula is indicated1–4 in formula fed babies.
- The only whey-based eHF that is both Halal and Kosher certified.
- Althéra is the only eHF to show similar efficacy* to an amino acid formula**(AAF). EHFs are not recommended where only an AAF is indicated.1,2
- Supports normal growth and development1,2
- Preferred taste over other casein based eHF which may help feeding acceptance, tolerance and health economic savings2,6-8
- Nutritionally complete. Suitable as a sole source of nutrition from birth or supplementary feeding from 6 months of age
*In a RCT
**in mild-severe cows’ milk allergy (CMA)
INGREDIENTS
Lactose (milk), maltodextrin, vegetable oils (sunflower, rapeseed, coconut), hydrolysed whey protein (milk), minerals (calcium glycerophosphate, potassium phosphate, magnesium chloride, calcium chloride, sodium chloride, potassium chloride, ferrous sulphate, sodium phosphate, zinc sulphate, potassium citrate, copper sulphate, manganese sulphate, potassium iodide, sodium selenate), emulsifier (E472c), Mortierella alpina oil (ARA), oil from the microalgae Schizochytrium sp. (DHA), choline bitartrate, acidity regulator (E 330), vitamins (C, E, niacin, pantothenic acid, riboflavin, A, thiamin, B6, folic acid, K, D, biotin, B12), L-arginine, L-histidine, taurine, inositol, L-carnitine.
PRESENTATION
400g tin (with a 4.4g scoop).
Precautions
- Use under strict medical supervision after full considerations of all the feeding options available, including breastfeeding.
- For oral or enteral use only.
- Not suitable for galactosemia, glucose-galactose malabsorption, lactose intolerance
- Althéra® is not recommended where only an amino acid formula is indicated.
ALLERGIES
Contains Milk
IMPORTANT FEEDING INFORMATION
Do not add any extra powder or water to make feeds stronger or weaker. Using too much or too little powder can make a baby ill. We recommend preparing each feed in individual bottles when required, feed immediately. If there is any unfinished infant milk left after feeding, throw it away. Always hold the baby while feeding. Never leave the baby alone during feeding as there is a risk they might choke.
SHELF LIFE AND STORAGE
Shelf life of 24 months from date of manufacture when stored at room temperature.
Once opened, reseal & store in a cool dry place and use within 3 weeks.
Important Notice
We believe that breastfeeding is the ideal nutritional start for babies as breast milk provides a balanced diet and protection against illness for a baby. We fully support the World Health Organisation’s recommendation of exclusive breastfeeding for the first six months of life followed by the introduction of adequate nutritious complementary foods along with sustained breastfeeding up to two years of age. We also recognise that breastfeeding may not be an option due to certain medical conditions. Parents should only feed infant formula for special medical purposes under supervision of a healthcare professional after full consideration of all feeding options, including breastfeeding. Continued use has to be assessed by the healthcare professional in relation to the baby’s progress bearing in mind any social and financial implications for the family. Infant formula should always be prepared, used and stored as instructed on the label in order to avoid risks to a baby’s health. Food for special medical purposes intended for infants must be used under medical supervision. For oral/ enteral use only. Suitable as a sole source of nutrition from birth or supplementary feeding from 6 months and up to 3 years of age
Preparing Althéra® is very similar to how you prepare standard infant formula. The standard dilution of 13.2% is made by adding 1 level scoop of Althéra® (approx 4.4g) to 30ml of water (approx 1 fluid oz). The step by step instructions on how to prepare Althéra® are:
STEP 1
Wash hands well.
STEP 2
Wash bottle, teat and cap.
STEP 3
Sterilise equipment (by boiling for 5 minutes or with a cold water sterilising solution).

STEP 4
Boil drinking (tap) water. Allow to cool for no more than 30 minutes.
STEP 5
Pour exact amount of water (see feeding table), into the sterilised bottle, carefully to avoid scalds, the water is hot.
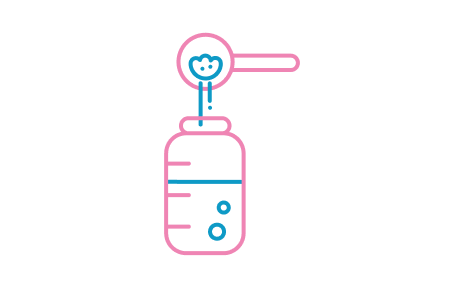
STEP 6
Add exact number of level scoops for age of baby (see feeding table), levelling off each scoop with the back of a clean dry knife. Store the scoop in suspension inside the tin and replace the lid.
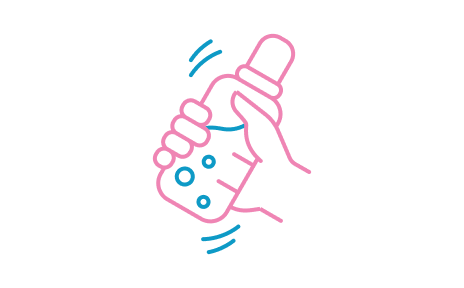
STEP 7
Place the sterilised teat and cap on the bottle and shake well until powder is fully dissolved. Cool bottle under cold running water until lukewarm. Test the temperature by shaking a few drops onto the inside of your wrist.
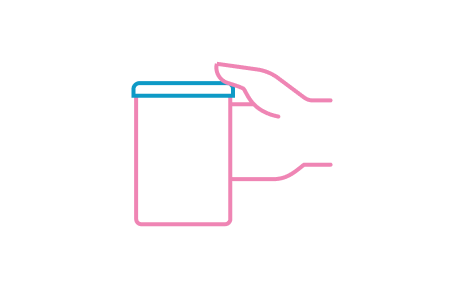
STEP 8
Close tin tightly after each use and store in a cool, dry place. Use within 3 weeks of opening.
WARNING:
“Unboiled water, unsterilsed bottles or incorrect dilution can make your baby ill. Incorrect storage, handling, preparation and feeding can also lead to adverse effects. Follow instructions carefully.”
Directions for use:
- Only prepare one bottle at a time.
- Clean the area where you are going to prepare the bottle.
- Feed immediately and follow the instructions exactly.
- Do not keep unfinished bottle, discard unused portion.
- Always hold baby while feeding.
- Leaving baby unattended may cause choking.
FEEDING TABLE:
| Age of Infant | Quantity per meal | Meals Per Day | |
|---|---|---|---|
| Water (ml) | No. of scoops* | ||
| 1-2 weeks | 90 | 3 | 6 |
| 3-4 weeks | 120 | 4 | 5 |
| 2nd month | 150 | 5 | 5 |
| 3-4 months | 180 | 6 | 5 |
| 5-6 months | 210 | 7 | 5 |
| From 6 months** | 210 | 7 | 3-4 |
* Note: Use only the enclosed scoop (4.4g).
** A healthcare professional should be consulted before introducing other food to a baby’s diet.
| Per 100g as sold | Per 100ml (standard 13.2% dilution) | |
|---|---|---|
| Energy kJ/ kcal | 2118 /506 | 280 /67 |
| Fat g (46% kcal) of which: | 26 | 3.4 |
| saturates g | 6.0 | 0.79 |
| monounsaturates g | 14 | 1.8 |
| polyunsaturates g | 4.4 | 0.58 |
| α-Linolenic acid mg | 380 | 50 |
| DHA mg | 130 | 17 |
| Linoleic acid mg | 3700 | 488 |
| ARA mg | 130 | 17 |
| Carbohydrate g (44% kcal) of which: | 55 | 7.3 |
| Sugars g | 29 | 3.8 |
| Lactose g | 28 | 3.7 |
| Protein g (10% kcal) | 12 | 1.6 |
| Salt g | 0.49 | 0.063 |
| Vitamins | ||
| A µg | 500 | 66 |
| D µg | 12 | 1.6 |
| K µg | 45 | 5.9 |
| C mg | 80 | 11 |
| Thiamin mg | 0.51 | 0.067 |
| Riboflavin mg | 1.0 | 0.13 |
| B6 mg | 0.40 | 0.053 |
| Niacin mg/mg NE | 7.0/12 | 0.92/1.6 |
| Folic acid µg | 75 | 9.9 |
| Folate µg DFE | 120 | 16 |
| B12 µg | 1.4 | 0.18 |
| Pantothenic acid mg | 3.3 | 0.44 |
| Biotin µg | 12 | 1.6 |
| E mg α-TE | 14 | 1.8 |
| Minerals | ||
| Sodium mg/mmol | 190/8.3 | 25/1.1 |
| Chloride mg/mmol | 400/11 | 53/1.5 |
| Potassium mg/mmol | 610/16 | 80/2.1 |
| Calcium mg/mmol | 530/13 | 70/1.7 |
| Phosphorus mg | 350 | 46 |
| Phosphate mmol | 11 | 1.5 |
| Magnesium mg/mmol | 45/1.9 | 5.9/0.24 |
| Iron mg | 5.0 | 0.66 |
| Zinc mg | 5.0 | 0.66 |
| Copper mg | 0.41 | 0.054 |
| Selenium µg | 25 | 3.3 |
| Chromium µg | <51 | - |
| Molybdenum µg | <71 | - |
| Iodine µg | 122 | 16 |
| Manganese mg | 0.068 | 0.009 |
| Fluoride mg | <1.0 | - |
| Other | ||
| MCT g | 3 | 0.4 |
| Taurine mg | 40 | 5.3 |
| L-Carnitine mg | 8.5 | 1.1 |
| Choline mg | 145 | 19 |
| Inositol mg | 35 | 4.6 |
| Osmolarity | 260 mOsm/l | |
| Osmolarity | 290 mOsm/kg | |
NE - Niacin Equivalent
TE - Tocopherol Equivalent
DFE - Dietary Folate Equivalent
DHA - Docosahexaenoic acid
ARA - Arachidonic acid
ACS015
Local guidelines9,10 describe an ideal prescribing ratio of 90% extensively hydrolysed formula and only 10% amino acid formula reserved for special cases.
ALTHÉRA® is the only eHF with the evidence1 and formulation5 to help you address your AAF prescribing imbalance.1,5,11,12
Getting you closer to an ideal ratio of 90% eHF: 10% AAF.10,13
Are you spending too much on cows’ milk allergy?
ALTHÉRA® IS THE ONLY EXTENSIVELY HYDROLYSED FORMULA TO SHOW SIMILAR EFFICACY† TO AN AMINO ACID FORMULA (AAF)* IN AN RCT WITH MILD-SEVERE CMA.1
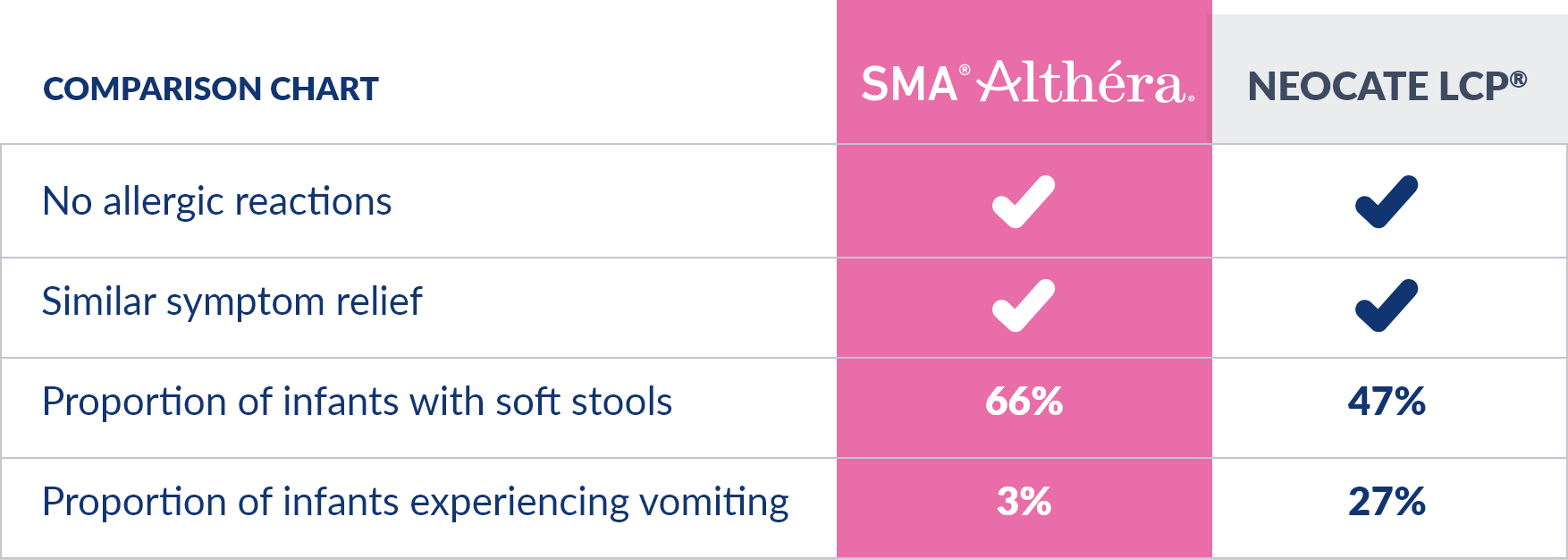
† In a randomized control trial
*SMA® Althéra® is not recommended where only an amino acid formula is indicated
Infants with CMA may face an immediate challenge from gastrointestinal, skin and/or respiratory symptoms.21-24 Althéra® is proven to be hypoallergenic, with a very low allergenic profile, as a result of Nestlé Health Science’s state-of-the-art hydrolysis process.1-5 Althéra® has been shown to effectively relieve infants from the symptoms of CMA1-4,6,7 as well as support normal growth.1,2,6,7,15-17

Skin symptoms
(e.g. eczema, urticaria…)

Gastrointestinal symptoms
(e.g. vomiting, colic, diarrhoea…)
Respiratory symptoms
(e.g. wheezing…)

General symptoms
(e.g. inconsolable crying, failure to thrive…)
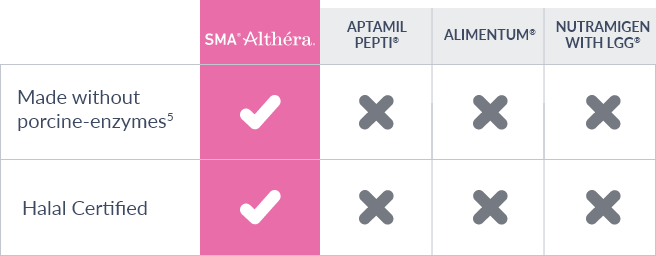
ALTHÉRA® is currently the only extensively hydrolysed formula (eHF) to utilise a plant and microbial derived enzyme blend as part of its hydrolysis process5. All other eHFs in the UK utilise a porcine (pig) enzyme in the manufacturing process.
The ALTHÉRA® formulation, made with the microbial and plant derived enzyme blend has proven effective in two RCT’s and demonstrated no difference in allergenicity vs the previous ALTHÉRA® formulation which used porcine derived enzymes.11,15
As a result of this change, ALTHÉRA® is now the only eHF in the UK suitable for halal diets and is the only Halal certified and vegetarian eHF in the UK.
Previously if you were unable to consume porcine-based products (either for dietary or religious reasons). you would have been offered an Amino Acid Formula as the only solution to meet your CMA needs.

The degree of hydrolysis and residual allergen content varies considerably between marketed casein- and whey-based eHF.16,17 These differences in peptide composition and residual allergen content impact on the clinical efficacy and risk of allergic reactions to eHF.17
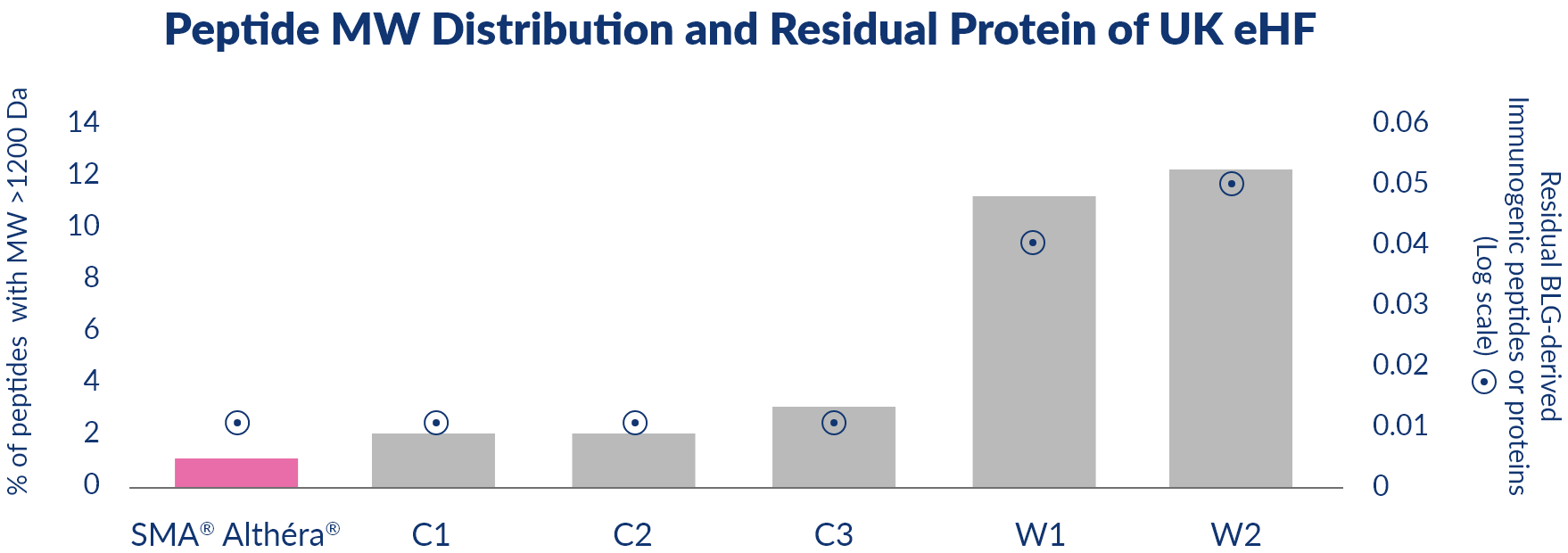
Graph adapted from Nutten et al17
C1 = Nutramigen LGG® 1, C2 = Nutramigen LGG® 2, C3 = Similac®
Alimentum, W2 = Aptamil™ Pepti 1, W1 = Aptamil™ Pepti 2
Da = Daltons MW = Molecular Weight BLG = Beta-lactoglobulin
The absence of food allergens in the form of intact proteins is a key feature of extensively hydrolysed
protein formulas; complex formulas, composed from many different ingredients from biotechnological or
agricultural origin, require a total quality management approach or ‘safety by design’ to ensure batch
to batch consistency and the safety of the product.
Althéra’s ingredients are selected only if they are safe for use in CMA management. The milk proteins
are broken down so that the peptide containing allergens are less likely to cause an allergic reaction.
To ensure that no larger protein fragments capable to cause an allergic reaction to remain in the finished
product, the hydrolysate is filtrated through several types of membranes including ultrafiltration with a
specific molecular weight cut off. The production process applied to Althéra is to maximise safety and
avoid any cross contamination with strict analytical controls in place in order to guarantee the absence
of intact proteins in the finished product.
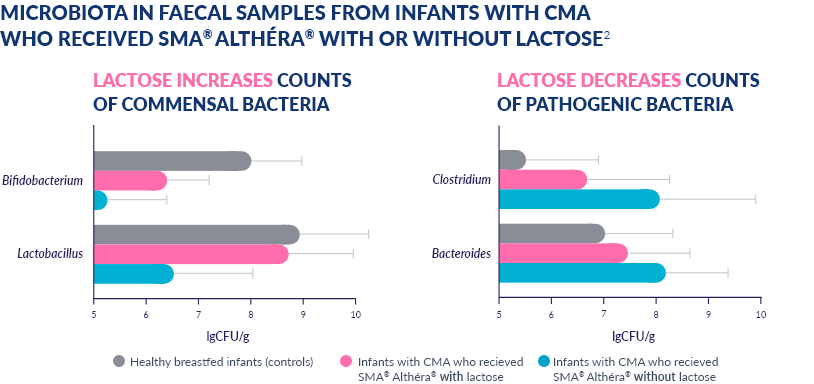
SMA® ALTHÉRA®: CONTAINS LACTOSE BECAUSE IT IS THE PRIMARY CARBOHYDRATE IN BREAST MILK19
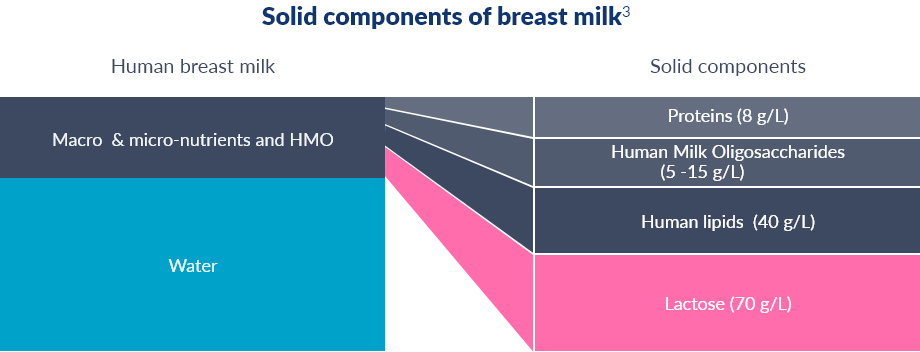
A 2012 STUDY SHOWED LACTOSE IN SMA® ALTHÉRA® POSITIVELY MODULATES THE GUT MICROBIOTA18-20
LACTOSE IN SMA® ALTHÉRA® CONTRIBUTES TO ITS BETTER TASTE
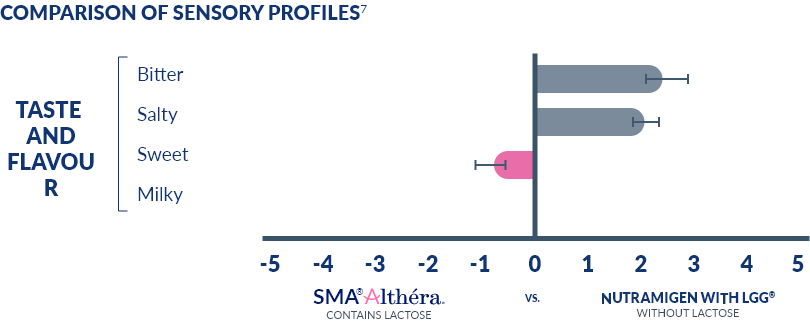
Lactose is a sugar and its “presence has been shown to make these naturally strong, bitter tasting extensively hydrolysed infant formulas better tasting”20
This may help feeding acceptance and health economic savings2,8,21-22
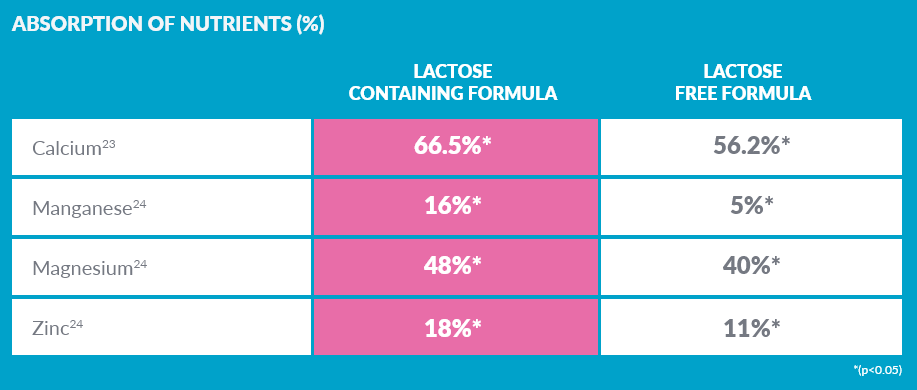
LACTOSE IN SMA® ALTHÉRA® MAY HELP TO INCREASE MINERAL ABSORPTION8,9
EHFs CONTAINING LACTOSE ARE RECOMMENDED BY
GUIDELINES AND SUPPORTED BY EXPERT OPINION

Frequently asked questions

Park House South
Crawley Business Quarter
Manor Royal
Crawley
RH10 9AD
Lactose intolerance results from a decreased ability to digest and absorb lactose (the sugar present in mammalian milk) due to a lack of the enzyme lactase. It is very rare in infants and children younger than 5 years28, even in those with CMA.
Breast milk naturally contains a high amount of lactose, which is beneficial for healthy infant growth and development29
| CMA Prevelance | Lactose intolerance Prevelance |
|---|---|
| 2.3% of infants ≤ 1 year31 | Very rare in children ≤ 5 years28
65% of people globally have lactose intolerance in adulthood30 |
Nestle Health Science - Cows’ Milk allergy
• Nestle Health Science - What is Cows' Milk Allergy
NICE Clinical Knowledge Summary
• Cow's milk allergy in children | Health topics A to Z | CKS | NICE
NICE Clinical Guidelines Food Allergy in Children and Young People
• Overview | Food allergy in under 19s: assessment and diagnosis | Guidance | NICE
Allergy UK
• Allergy Symptoms and Conditions | Allergy UK
British Society of Allergy and Clinical Immunology
• Cow's Milk Allergy – BSACI
Anaphylaxis Campaign
• https://www.anaphylaxis.org.uk/information-training/facts-and-figures/
| Product | Product Format | PIP Code | Nestle Line Code | Case Size |
|---|---|---|---|---|
| SMA® ALTHÉRA® | 400 g Tin | 413-8269 | 12287423 | 6 |
OTHER PRODUCTS IN OUR RANGE OF SPECIALIST FORMULAS FOR COW’S MILK ALLERGY
References:
- Niggemann B et al. Pediatr Allergy Immunol 2008; 194(4): 348–354.
- Vandenplas Y et al. Acta Paediatr 2013; 102(8): 990–998.
- Meyer R et al. EMJ Allergy and Immunol 2017; 2(1): 41–51.
- Madrazo-de la Garza J et al. EMJ Allergy and Immunol. 2018; 31(1): 56–58.
- Information from product data cards; Nutramigen with LGG, Alimentum, Aptamil Pepti, Aptamil Syneo. July 2021
- Data on File. Althéra® versus casein-based formulas. Independent competitive benchmarking test.
- Rapp M et al. Poster presentation: Food Allergy and Anaphylaxis Meeting (FAAM 2013) Nice, France. 7-9 February 2013.
- Maslin K. et al. Pediatr Allergy Immunol 2018; 29(8): 857–862.
- Greater Manchester Joint Commissioning Team. Prescribing infant formula for cow’s milk protein allergy in primary care. Version 3. April 2020. Available from: http://gmmmg.nhs.uk/docs/guidance/GMMMG-CMPA-final-3-0.pdf
- Midlands and Lancashire CSU. Prescribing Guidelines for Specialist Infant Formula Feeds. December 2017. Available from: https://www.lancsmmg.nhs.uk/media/1430/prescribing-guidelines-for-specialist-infant-formula-feeds-v31-final-for-website.pdf
- Nowak-Wegrzyn A, et al. Allergy. 2019; 74:1582-4
- Guidance for Health Professionals on safe preparation storage and handling of powdered infant formula. 2007. Food Standards Agency. Department of Health. UK. Available from http://www.foof.gov.uk.
- Data on File. EHF used for calculation is SMA Althera (vs. Aptamil Pepti, Nutramigen with LGG & Similac Alimentum) & AAF used for calculation is SMA Alfamino (vs. Neocate LCP, Neocate Syneo, Neocate Junior, Elecare and Puramino). MAT to June 2020.
- MIMS, accessed July 2021
- Vanderplas Y et al. Abstract. Pediatric Allergy and Asthma Meeting 2019, Florence, Italy.
- Lambers, TT. Et al. Food Sci Nutr. 2015;3:81-90
- Nutten, S. et al. Allergy. 2020. Jun; 75(6):1446-1449
- Francavilla, R. et al. Pediatr allergy Immunol. 2012; 23:420-427
- O’Mahony, L. et al. EMJ Allergy and Immunol. 2018; 31(1):56-58.
- Waddell, L. Clinical Nutrition 2016;15(6):23-28
- Luyt, D. et al. Clin Exp Allergy, 2014; 44:642-672.
- Rapp, M. et al. Clin Transl Allergy, 2013; 3(3):132
- Abram, S. et al. Am J Clin Nutr. 2002; 76(2):442-446
- Ziegler, E & Fomon, J. J Pediatr Gastroenterol Nutr, 1983; 288-294.
- CODEX ALIMENTUS: Standard for infant formula and formulas for special medical purposes intended for infants. 2007. (accessed January 2020; www.fao.org)
- Koletzko, S. et al. J Pediatr Gastroenterology Nut, 2012; 55: 221-229.
- Paoli G. Harthett E. Overview of a risk assessment model for Enterobacter sakazahii in Powdered Infant Formula.2007. Joint FAO/WHO Expert Meetings on Microbiological Risk Assessment (JEMRA). Available from: www.who.int/foodstaftey/micro.jemra.
- Heyman MB et al. Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118(3):1279–86.
- Martin C et al. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279
- NIH. Lactose intolerance. Available at: https://ghr.nlm.nih.gov/condition/lactose-intolerance#statistics (accessed April 2021).
- Venter, C., Pereira, B., Voigt, K. et al. (2008) Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy 63(3), 354-359
- Koletzko B et al, Am J Clin Nutr, 2009. 89: p. 1836-1845.
- Koletzko B et al, Am J Clin Nutr, 2009. 85: p. 1502S-1508S.
FOOTNOTES & ABBREVIATIONS
AAF, amino acid formula
CMA, cows’ milk allergy
eHF, extensively hydrolysed formula
FAQs, frequently asked questions
MFPA, multiple food protein allergies
WHO, World Health Organisation
DOH, Department of Health
Nestlé Health Science produces a range of foods for special medical purposes (FSMP) for use under medical supervision. A sample product should only be requested for a patient if deemed suitable following a professional evaluation from an appropriate healthcare professional. Althera® and Alfamino® are infant FSMPs and should only be used after the full consideration of the feeding options available, including breastfeeding.
The Nestlé Health Science Sample Service is available to healthcare professionals only, who require sample products for professional evaluation when they have no sample product or if a patient has insufficient product to cover their needs. This service is not intended as a long term solution for a patient.
® Reg. Trademark of Société des Produits Nestlé S.A. Terms and conditions apply.
IMPORTANT NOTICE: Mothers should be encouraged to continue breastfeeding even when their babies have cows' milk protein allergy. This usually requires qualified dietary counselling to completely exclude all sources of cows' milk protein from the mothers’ diet. If a decision to use a special formula intended for infants is taken, it is important to follow the instructions on the label. Unboiled water, unboiled bottles or incorrect dilution can make babies ill. Incorrect storage, handling, preparation and feeding can eventually lead to adverse effects on the health of babies. Formula for special medical purposes intended for infants must be used under medical supervision.

Nestlé Health Science UK Statement on Demand for Specialist Infant Formulas for Cows’ Milk and Multiple Food Allergies
We believe breast milk is the best food for infants. When in consultation with their healthcare professional, mothers and families find that optimal breastfeeding is not possible due to their infant’s medical condition, formulas for special medical purposes play a vital role in providing essential nutrients to infants. We have a global commitment to market breast-milk substitutes responsibly.
This website is about the management of cows’ milk protein allergy and nutritional solutions intended for infants. By continuing on this website, you accept that Nestlé Health Science supplies the information at your own request.
Are you a healthcare professional (HCP) or a parent?




