
We believe breast milk is the best food for infants. When in consultation with their healthcare professional, mothers and families find that optimal breastfeeding is not possible due to their infant’s medical condition, formulas for special medical purposes play a vital role in providing essential nutrients to infants. We have a global commitment to market breast-milk substitutes responsibly.
This website is about the management of cows’ milk protein allergy and nutritional solutions intended for infants. By continuing on this website, you accept that Nestlé Health Science supplies the information at your own request.
Are you a healthcare professional (HCP) or a parent?
OUR RANGE OF HYPOALLERGENIC CMPA TREATMENTS: ALTHÉRA® AND ALFAMINO®
Nestlé Heath Science's Althéra® and Alfamino® have been developed as a cows’ milk allergy treatment to help meet the growth and development needs of infants and children with food allergies and intolerances.1,2
NUTRITIONAL SOLUTIONS FOR COWS’ MILK PROTEIN ALLERGY TREATMENT
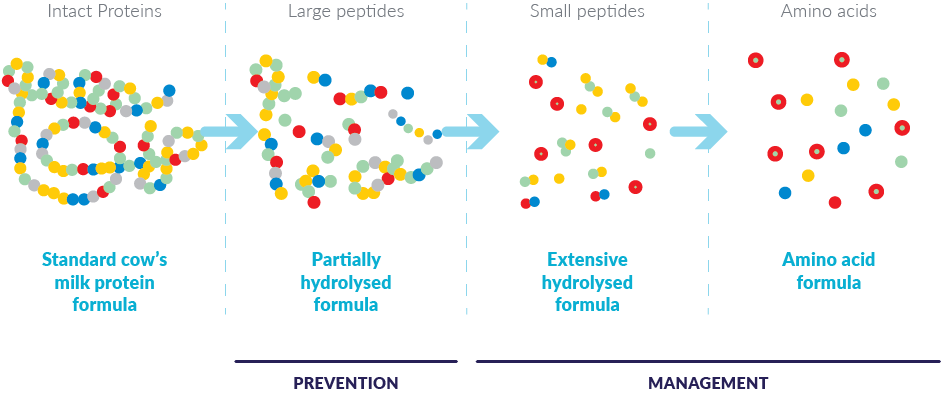
Following diagnosis, CMA is managed through the complete removal of cows' milk proteins (CMPs) from the infant’s diet. CMA can also occur among exclusively breastfed infants. When an exclusively breastfed infant is diagnosed with CMA, the mother must exclude all CMP from her diet.3
Mothers should be encouraged to continue breastfeeding, but in case this is not an option, specialist infant formulas are available for infants and young children with CMA to reduce or completely remove the allergenic potential.3 These formulas contain the nutrients to help support healthy infant growth and development.1,2 There are two different types of formulas for the management of CMA:
Extensively hydrolysed formulas,* or “eHFs,” in which CMPs have been extensively hydrolysed into peptides. As a result, they are less allergenic than whole milk formulas and are well tolerated by most children with cows' milk or soy protein allergies.3-5
Amino acid-based formulas (AAFs) contain free amino acids, which are non-allergenic. They are recommended when an extensively hydrolysed formula is not tolerated or as first-line dietary management when the baby has extremely severe or life-threatening allergic symptoms (anaphylaxis or immediate type reactions).3-5
*Note: Partially hydrolysed formulas (pHFs) are also sometimes referred to as hypoallergenic; however, pHFs are not intended for dietary management of diagnosed CMA.
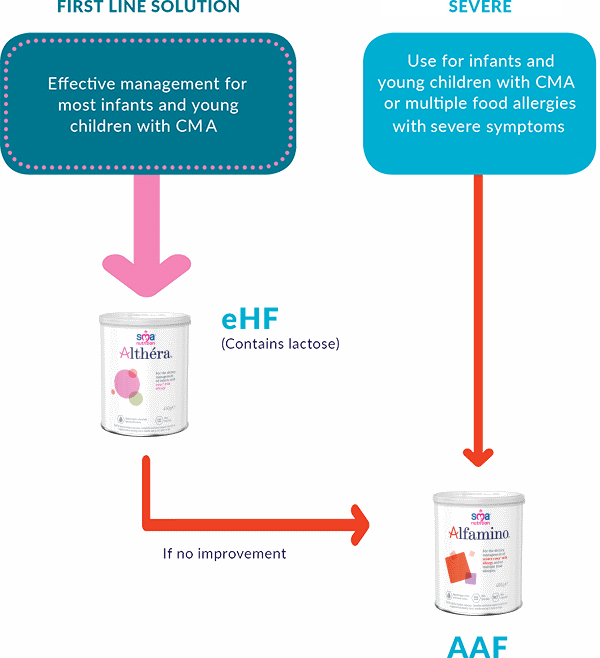
CHOOSING THE RIGHT NUTRITIONAL SOLUTION
Mothers should be encouraged to continue breastfeeding, however if this is not an option, this decision tree will help you to consider the possible cows’ milk protein allergy treatment option for your patients based on their symptoms and will help you in selecting the right solution at the right time.
A product characterisation analysis presented at the EAACI 2017 annual congress compared eHF samples using the same analytical methods. It compared peptide size and residual protein content and showed that eHFs may have different allergenic potentials.10,11 Read more here.
Safety by design is the leading principle in the development of Nestlé Health Science’s product portfolio. Safety is a fundamental consideration for developing all formulas, from the formula design and manufacturing set-up to clinical testing and production. In the development of extensively hydrolysed hypoallergenic formulas for use as a CMPA treatmen, one of the fundamental processes is the removal of allergenic epitopes so that they no longer cause an immune reaction. Epitope removal is achieved through hydrolysis, after which post-hydrolysis processing, e.g. ultrafiltration, takes place.
Studies have demonstrated the safety of Althéra® and Alfamino® in cases of CMA and/or multiple food allergies in infants and young children.1,2,6
Hydrolysis is an important procedure in the breakdown of antigens or allergens that cause allergic reactions. The production of a hypoallergenic formula is based on the destruction of cows' milk protein epitopes through different technologies (heat treatment and extensive enzymatic hydrolysis). Increasing the degree of hydrolysis of milk proteins results in the removal of the larger peptides, which significantly helps to reduce the allergenicity of the formulas.3 Althéra® has the least amount of peptides above 1200 Daltons compared with competitors.6
Nutrition plays an essential role for healthy development during the first years of life. Lactose is an important component of breast milk, representing up to 40% of the total energy content.7 Lactose is important for supporting calcium absorption and inhibiting putrefactive bacteria in the gut, which promotes the growth of healthy gut microbiota.8 Lactose also has another benefit; it improves the taste of these specialist formulas, facilitating acceptance for infants.
Nestlé Health Science not only develops products that are hypoallergenic/non-allergenic and helps to support growth and development1,2, but we also place a high priority on creating products that are neutral tasting. Easier acceptance, owing to pleasant taste and a natural milk aroma, most likely contributes to improved consumption of formula in the dietary management of food allergies such as CMA and/or multiple food allergies.9
- Niggemann B, et al. Safety and efficacy of a new extensively hydrolyzedhydrolysed formula for infants with cows’ milk protein allergy. Pediatr Allergy Immunol. 2008;19(4):348–54.
- Nowak-Wegrzyn A, et al. Evaluation of hypoallergenicity of a new, amino acid-based formula. Clin Pediatr (Phila). 2015;54(3) 264–72.
- Luyt D et al. BSACI Milk allergy guideline. Clinical & Experimental Allergy. 2014 (44) 642–672. 2008;19(4):348–54.
- Venter C et al. Clinical and Translational Allergy. 2013;3:23.
- NICE. Cows' milk protein allergy in children NICE CKS. 2015 Available at: https://cks.nice.org.uk/cows-milk-protein-allergy-in-children#!scenario:1 [Accessed Nov 2017].
- Rapp M, et al. Characterization of an extensively hydrolyzedhydrolysed whey infant formula with a low bitterness. Clin Transl Allergy. 2013; 3(3): P132.
- Infant nutrition and feeding. A guide for use in the WIC and CSF programs. March 2009:14.
- Francavilla R, et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr Allergy Immunol. 2012;23(5):420–7.
- Data on file. 2018.
- Meyer, R. et al. EMJ Allergy and Immunol. 2017; 2(1): 46–51.
- Nutten S, et al. Abstract. EAACI Congress, 26-30 May, 2018.
